Abstract
Background
Vaccination (vac) strategies to maintain remissions in AML have been pursued for decades. The usage of recombinant proteins instead of peptides allows a potential immune response to multiple epitopes, hence could be offered to all patients (pts) independent of HLA expression. Wilms' tumor 1 (WT1) protein is highly immunogenic and frequently overexpressed in AML, thus ranked as a very promising target for novel immunotherapies. Here we report a single-center experience of a phase I/II clinical trial (NCT01051063) of a first-in-human vac strategy based on WT1 recombinant protein (WT1-A10) together with vaccine adjuvant AS01 B in 5 elderly AML pts.
Patients and Methods
Key eligibility criteria: overexpression of WT1 transcripts in AML blasts at diagnosis (qRT-PCR); 1 or 2 induction chemotherapies, with partial remission (PR) or morphologic complete remission with incomplete blood count recovery (CRi). The vaccine consisted of WT1-A10, a truncated WT-1 protein retaining the N-terminus (amino acids 2-281) of full length WT1 protein (429 aa) linked to the first 11 amino acids of trimethylamine N-oxide reductase signal peptide via one histidine residue combined with the liquid AS01 B adjuvant. AS01 B is an Adjuvant System containing MPL (3-O-desacyl-4´-monophosphoryl lipid A), QS-21 (Quillaja saponaria Molina, fraction 21) and liposome (50µg MPL and 50µg QS-21). One human dose of WT1-A10 + AS01 B contained 200 μg of WT1-A10 antigen. Pts received the vaccine by i.m. injection. To assess cellular response, antigen-specific stimulation of cultured PBMCs was performed with a pool of 123 15mer peptides covering the entire WT1 (1μg/ml/peptide), together with irrelevant re-stimulation plus negative control peptide. CD4 + and CD8 + T cells were serially assessed by intracellular flow cytometry for their ability to produce both IFN-γ and TNF upon antigen stimulation.
Results
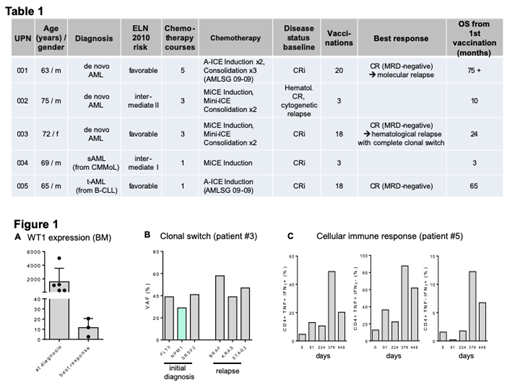
A total of 5 pts (median age 69, range 63-75) were enrolled on the WT1 protein-based vac study at our institution (Table 1), receiving a total of 62 vac after a median of 3 courses (1-5) of standard chemotherapy. The repeated vac had an acceptable safety profile and were thus well tolerated. 2/5 pts experienced therapy-related toxicity, injection site pain (CTCAE v.3, grade 2) and injection site inflammation (CTCAE v.3, grade 1). Symptoms were of mild / moderate severity and resolved completely. No hematologic toxicity was noted. With a median progression-free survival of 28.8 mths (range 1-59) and median overall survival (OS) of 35.4 mths (range 3-75) from the 1st vac, this older patient cohort showed above-average clinical outcome (Table 1), pointing to a potential clinical efficacy of WT1-based vac therapy. All vaccinated pts showed highly elevated WT1 ratios before WT1-based vac therapy and normal levels after vac (Fig. 1A). Two pts demonstrated early relapse after 3 WT1 protein-based vac, and clinical benefit was observed in 3 pts: one achieved complete and sustained measurable residual disease clearance (NPM1 ratios) during WT1 vac, resulting in molecular CR at the 18th vac. The pt died from unrelated reasons 5.5 years after initial diagnosis of AML, 3.5 years after the last WT1 vac, with continued molecular CR. One pt maintained long-term hematological and molecular remission over 59 mths, until molecular relapse occurred 11 mths after the final, 21 st vac. Interestingly, in one case, a complete clonal switch occurred at hematologic relapse following 18 vac, with loss of WT1 overexpression: while the clone at initial diagnosis harbored FLT3, NPM1 and SRSF2 mutations, BRAF, KRAS and STAG2 mutations were detected at relapse (Fig. 1B), pointing to an ongoing suppression of the WT1 expressing AML clone. Flow cytometry studies were conducted in one pt to elucidate specific cellular immune responses. We noted CD4 + T cell immune responses by strong IFN-γ and TNF expression (Fig. 1C), suggestive of efficient immune stimulation post-vac, while CD8 + T cells failed to upregulate these key cytokines.
Conclusions
This vac strategy showed good feasibility, with a very acceptable safety profile, and appeared to extend remissions beyond the expected duration, together with MRD clearance. Thus, our data provide evidence of potential clinical efficacy of WT1 protein-based vac therapy in AML pts, making this maintenance approach an attractive alternative to more complex strategies, particularly in elderly pts with comorbidities.
Döhner: Abbvie: Consultancy, Honoraria; Jazz Roche: Consultancy, Honoraria; Daiichi Sankyo: Honoraria, Other: Advisory Board; Janssen: Honoraria, Other: Advisory Board; Celgene/BMS: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Research Funding; Astellas: Research Funding; Agios and Astex: Research Funding. Schmitt: MSD: Membership on an entity's Board of Directors or advisory committees; TolerogenixX: Current holder of individual stocks in a privately-held company; Bluebird Bio: Other: Travel grants; Hexal: Other: Travel grants, Research Funding; Novartis: Other: Travel grants, Research Funding; Kite Gilead: Other: Travel grants; Apogenix: Research Funding. Lübbert: Teva: Research Funding; Janssen: Research Funding; Cheplapharm: Research Funding; Aristopharm: Research Funding; Syros: Honoraria; Pfizer: Honoraria; Janssen: Honoraria, Research Funding; Imago BioSciences: Honoraria; Hexal: Honoraria; Astex: Honoraria; Abbvie: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal